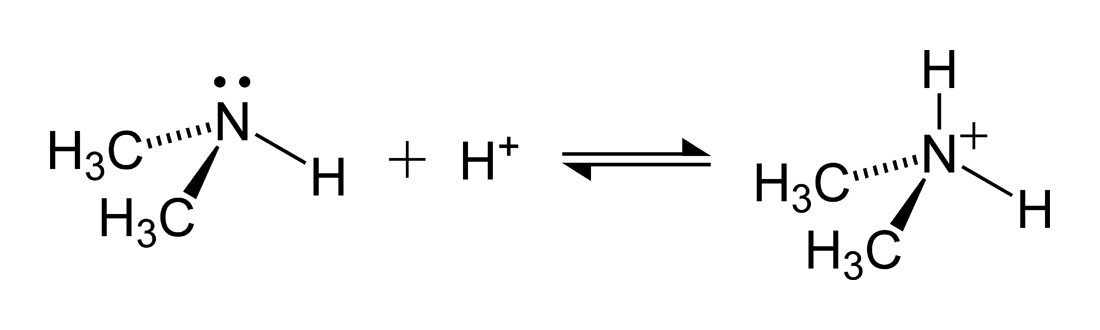Give reasons : (i) (CH3)2NH is more basic than (CH3)3N in an aqueous solution. - Sarthaks eConnect | Largest Online Education Community

SOLVED: 1.) A 21.4 mL sample of 0.271 M dimethylamine, (CH3)2NH, is titrated with 0.372 M nitric acid. After adding 23.5 mL of nitric acid, the pH is . 2.) A 29.4

14.37 | Which base, CH3NH2 or (CH3)2NH, is the stronger base? Which conjugate acid, (CH3)2NH2+ or - YouTube
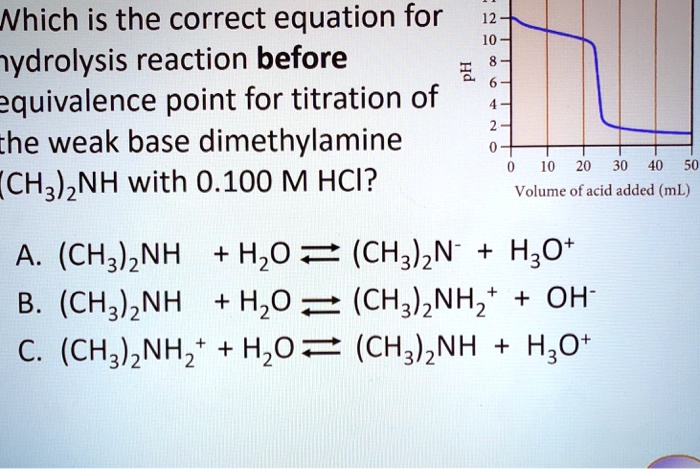
SOLVED: Nhich is the correct equation for ydrolysis reaction before 2 quivalence point for titration of he weak base dimethylamine (CHz)NH with 0.100 M HCI? Volume of acid added (mL) A (CH3)zNH

What is the order of basicity of the following compounds? CH3NH2, (CH3)2NH, (CH3)3N (in protic solvent)

SOLVED: For the following reaction, K < 1. Classify each of the reactants and products based on their strength as Bronsted-Lowry acids or bases. (CH3)2NH2+ + CN- –> (CH3)2NH + HCN CN-