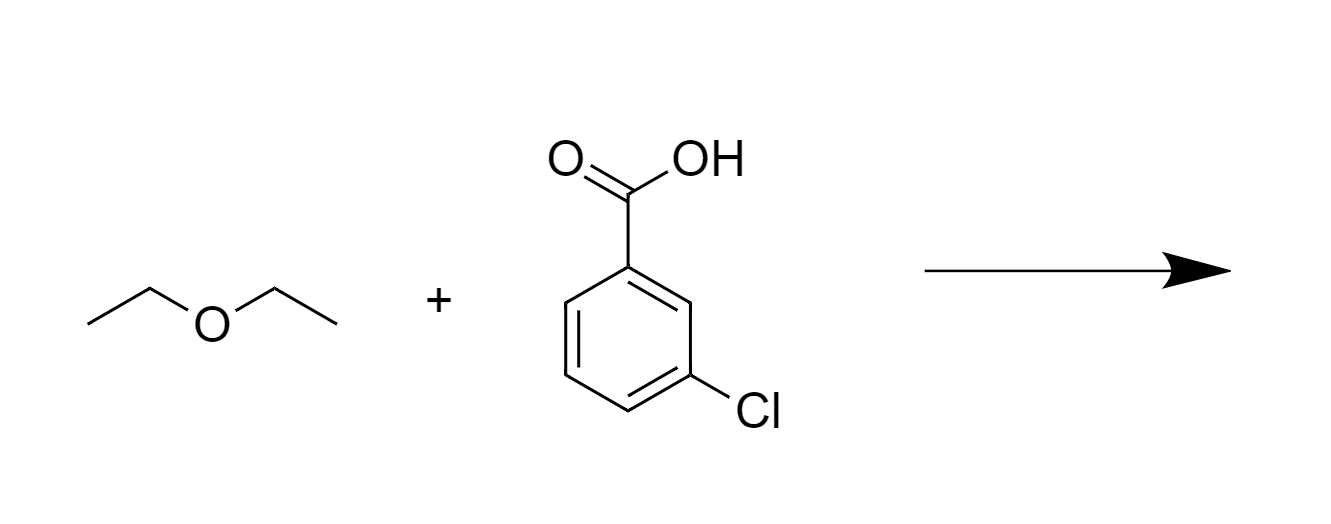
React diethyl ether with water in the presence of an acid. What is the reaction? A. oxidation B. hydrogenation C. ether hydrolysis D. dehydration | Homework.Study.com
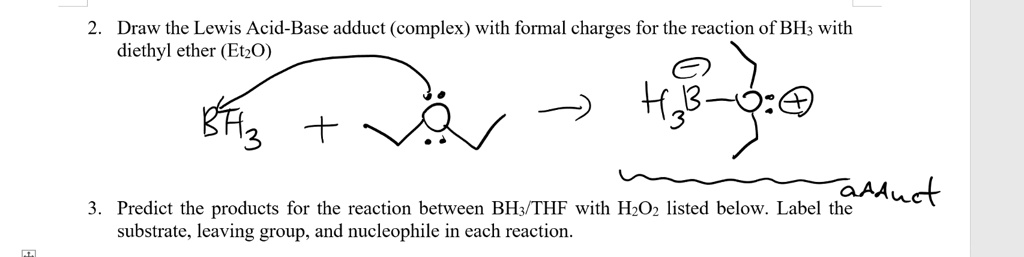
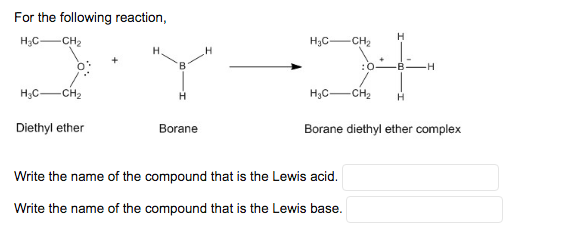
SOLVED: Draw the Lewis Acid-Base adduct (complex) with formal charges for the reaction of BH; with diethyl ether (EtzO) aAAuct Predict the products for the reaction between BH3 THF with HzOz listed

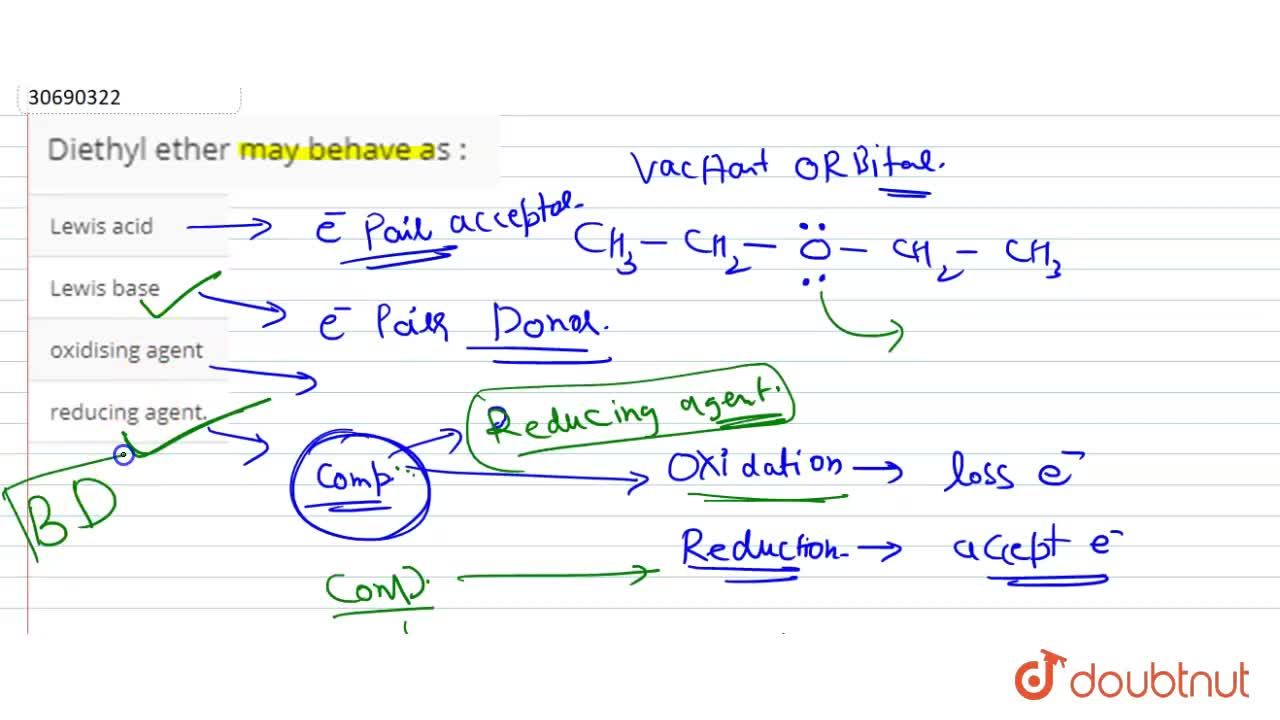
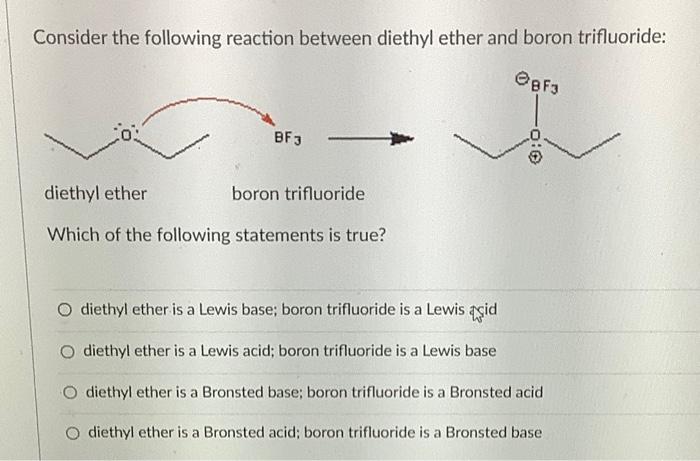
CH_3CH_2OCH_2CH_3 is best classified as a ______. A) Bronsted-Lowry acid. B) Lewis acid. C) Bronsted-Lowry base. D) Lewis base. E) Both C & D. | Homework.Study.com

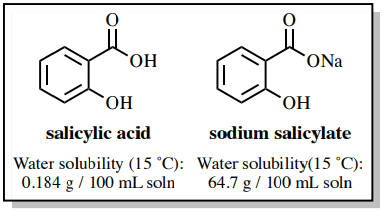
SOLVED: Phenol is soluble in diethyl ether but not water; however , phenol is extracted from diethyl ether with aqueous sodium hydroxide Complete the acid-base reaction below by writing the products of

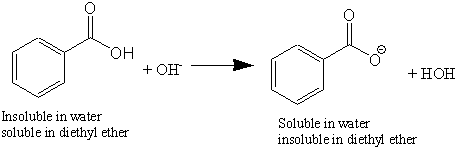
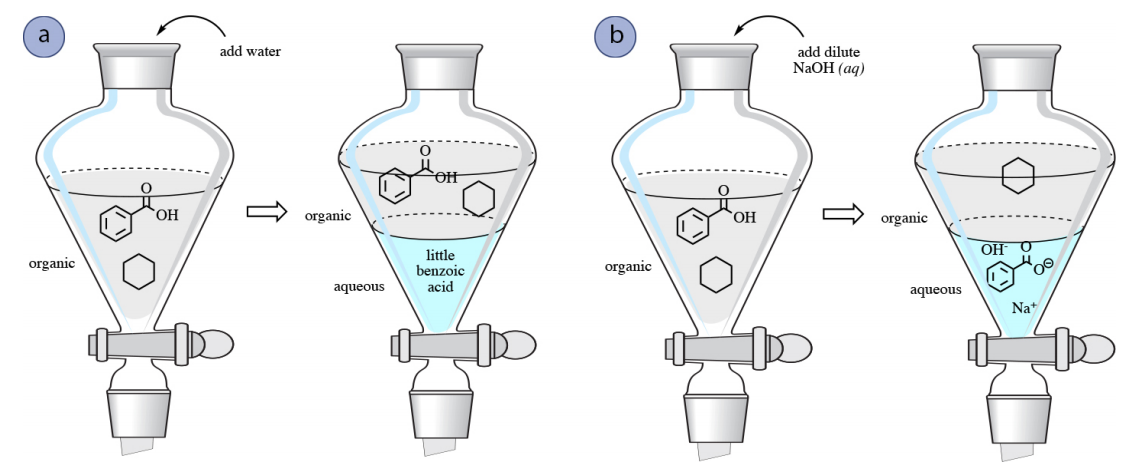
OneClass: Benzoic acid is soluble in diethyl ether but not water, however, benzoic acid is extracted ...