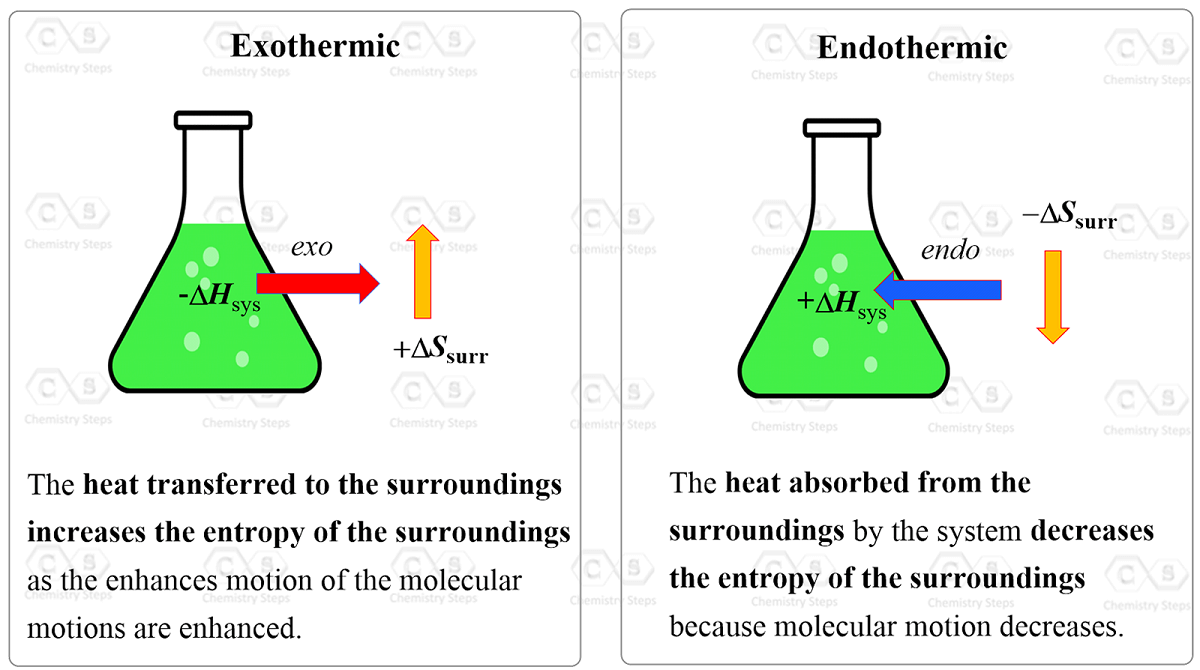
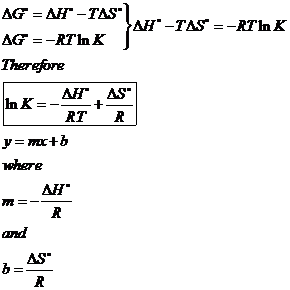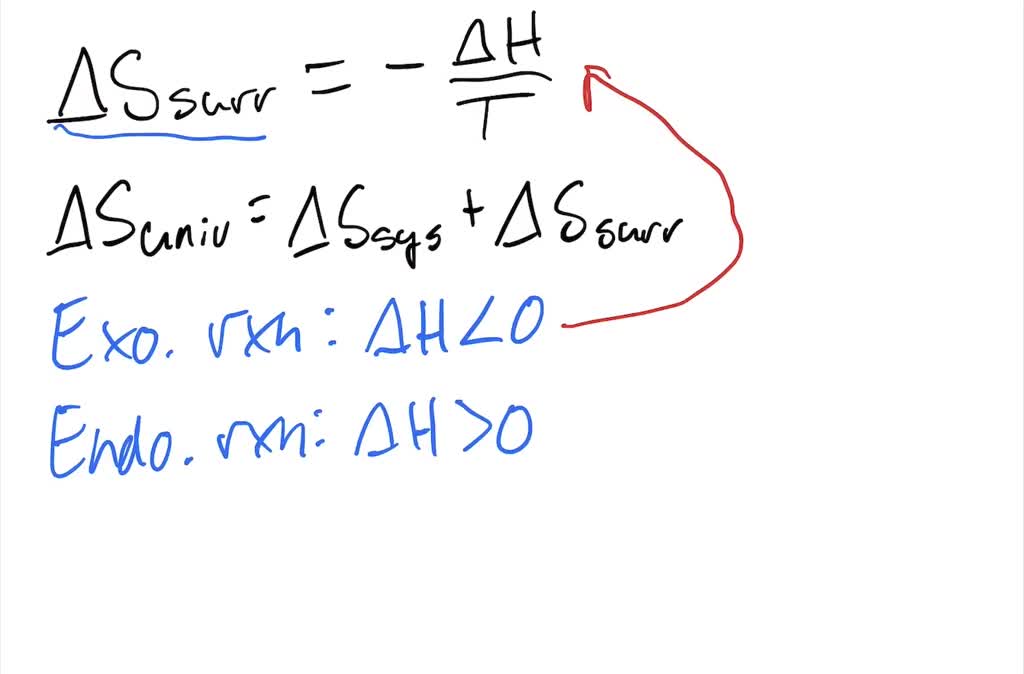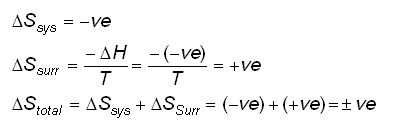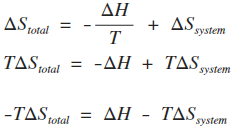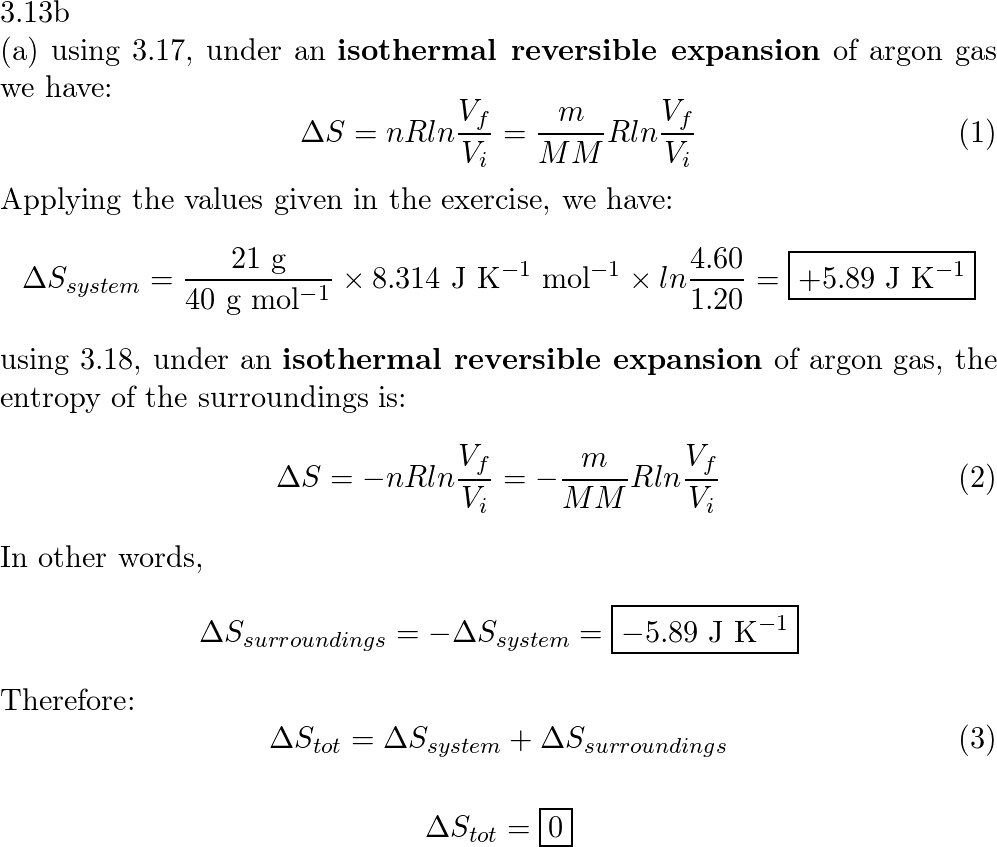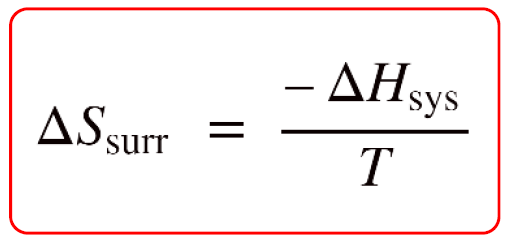SOLVED:Calculate ΔSsur for the following reactions at 25^∘ C and 1 atm. a. C3 H8(g)+5 O2(g) ⟶3 CO2(g)+4 H2 O(l) ΔH^∘=-2221 kJ b. 2 NO2(g) ⟶2 NO(g)+O2(g) ΔH^∘=112 kJ

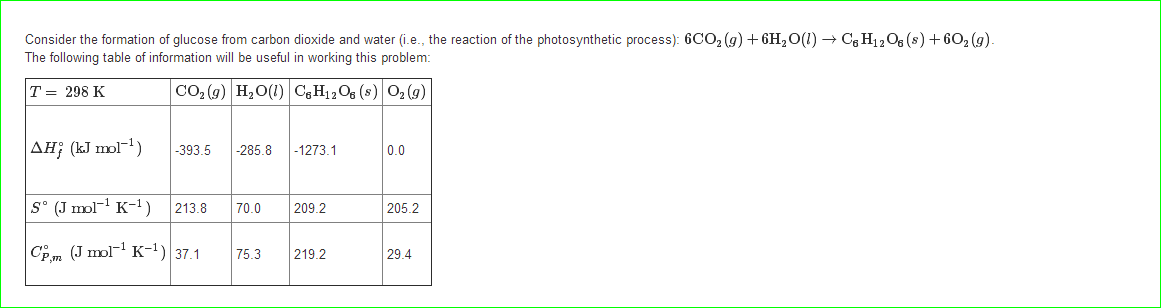
Calculate ΔS univ (in J/K) for the chemical reaction: C(graphite) + 2H2(g)→CH4(g);ΔH300^o = - 75.0kJ . The standard entropies of C(graphite),H2(g),CH4(g) are 6.0,130.6 and 186.2J/K - mol , respectively.
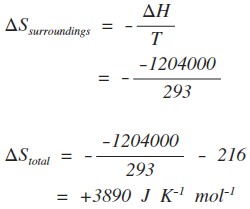
Calculate the entropy change in the system. and in the surroundings and the total entropy change in the universe when during - Sarthaks eConnect | Largest Online Education Community

physical chemistry - Why doesn't Delta S total = 0 for this reversible process? - Chemistry Stack Exchange