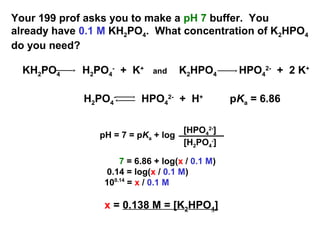
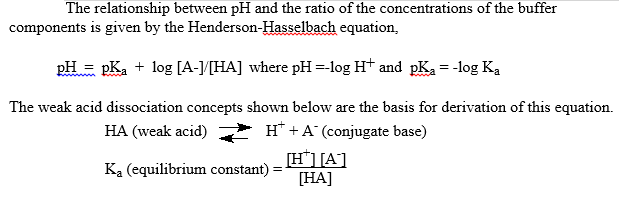
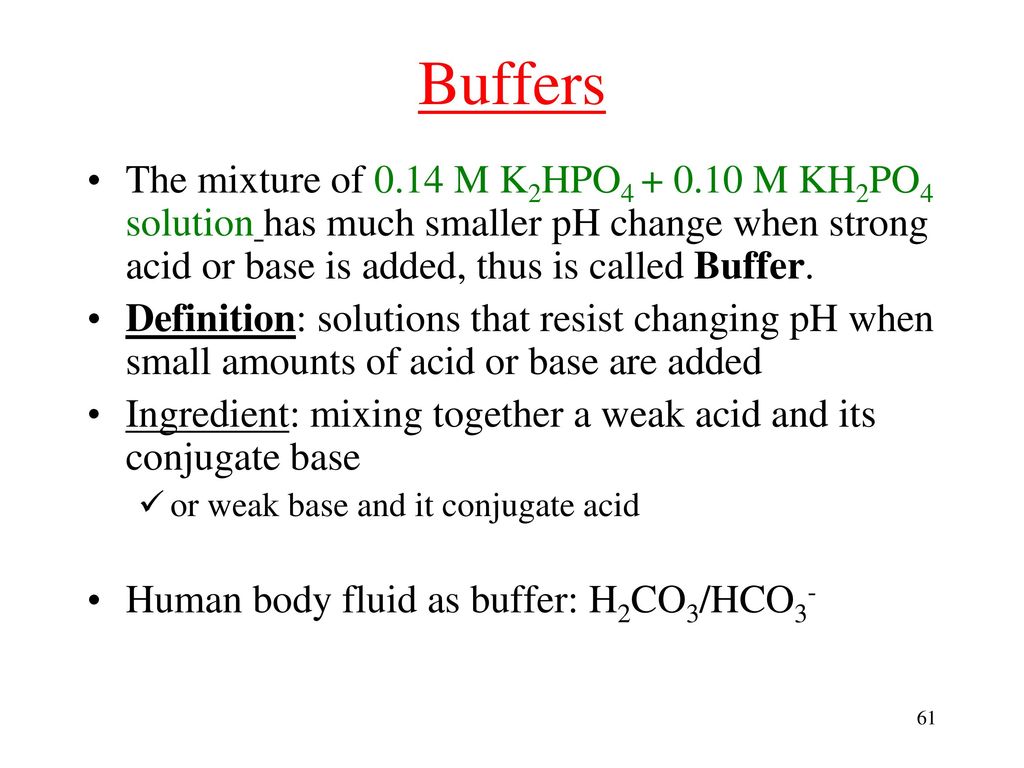
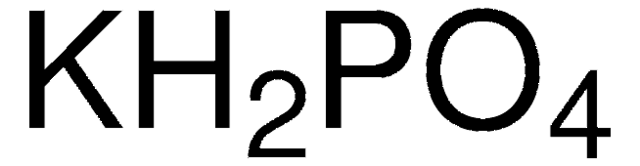![Potassium Phosphate Monobasic [KH2PO4] 99+% Fine Crystals 1.5 Lb in Three Space-Saver Bottles: Amazon.com: Industrial & Scientific Potassium Phosphate Monobasic [KH2PO4] 99+% Fine Crystals 1.5 Lb in Three Space-Saver Bottles: Amazon.com: Industrial & Scientific](https://m.media-amazon.com/images/I/515VzhubFGL._SR600%2C315_PIWhiteStrip%2CBottomLeft%2C0%2C35_PIStarRatingFOUR%2CBottomLeft%2C360%2C-6_SR600%2C315_ZA6%2C445%2C290%2C400%2C400%2CAmazonEmberBold%2C12%2C4%2C0%2C0%2C5_SCLZZZZZZZ_FMpng_BG255%2C255%2C255.jpg)
Potassium Phosphate Monobasic [KH2PO4] 99+% Fine Crystals 1.5 Lb in Three Space-Saver Bottles: Amazon.com: Industrial & Scientific
![Potassium Phosphate Monobasic [KH2PO4] 99+% Fine Crystals 1.5 Lb in Three Space-Saver Bottles: Amazon.com: Industrial & Scientific Potassium Phosphate Monobasic [KH2PO4] 99+% Fine Crystals 1.5 Lb in Three Space-Saver Bottles: Amazon.com: Industrial & Scientific](https://m.media-amazon.com/images/I/91efPWuddQL._AC_UF1000,1000_QL80_.jpg)
Potassium Phosphate Monobasic [KH2PO4] 99+% Fine Crystals 1.5 Lb in Three Space-Saver Bottles: Amazon.com: Industrial & Scientific

Enhanced Second-Harmonic-Generation Response in a KH2PO4-Type Calcium Nitrate Carboxylate with Unusual Three-Dimensional Inorganic and Organic Connections | Inorganic Chemistry

14.87 | Calculate the pH of a buffer solution prepared from 0.155 mol of phosphoric acid, 0.250 mole - YouTube