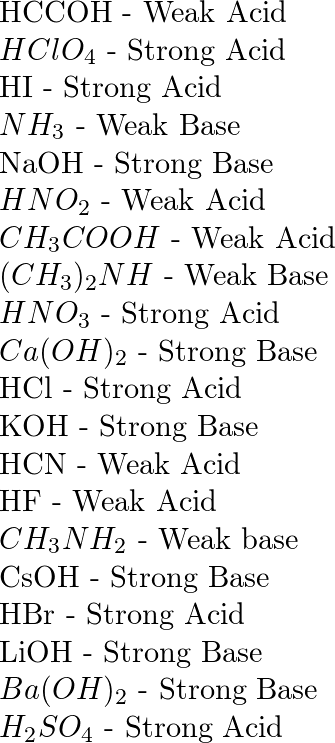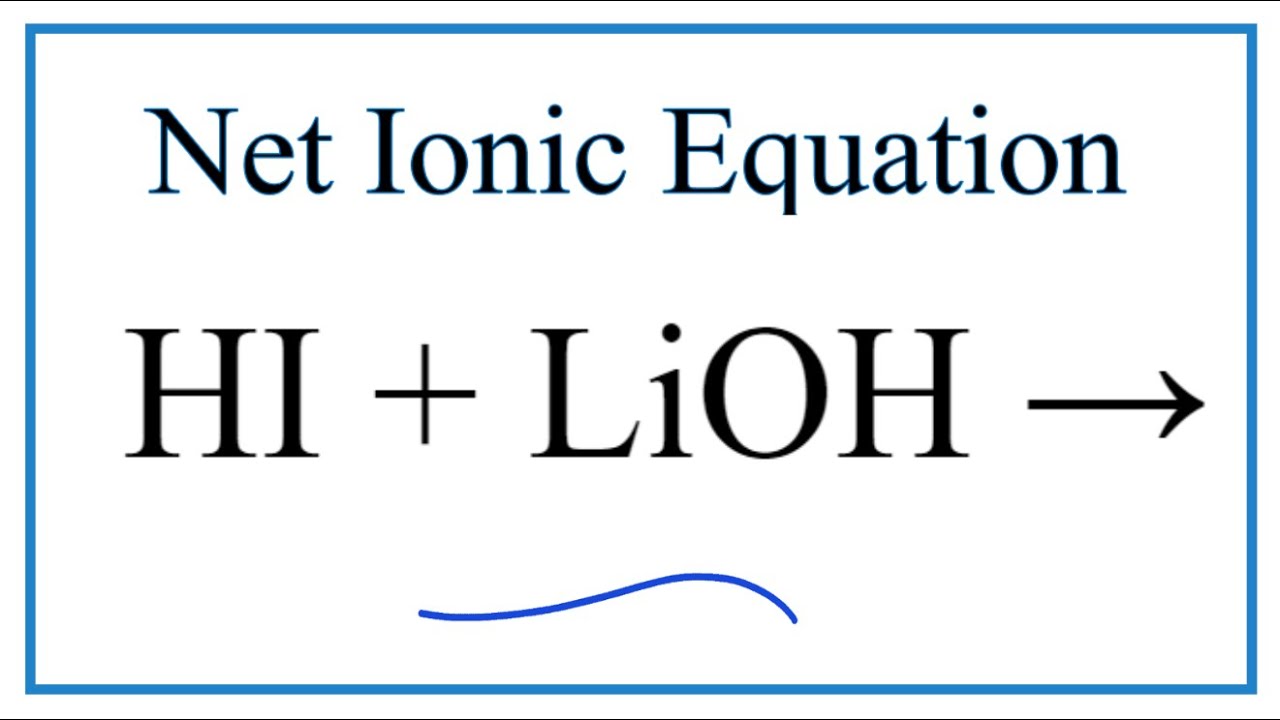Titration curves for solutions with 1.0 × 10 −4 moles of LiOH·H 2 O and... | Download Scientific Diagram
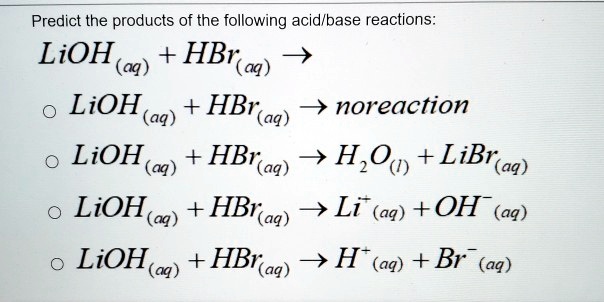
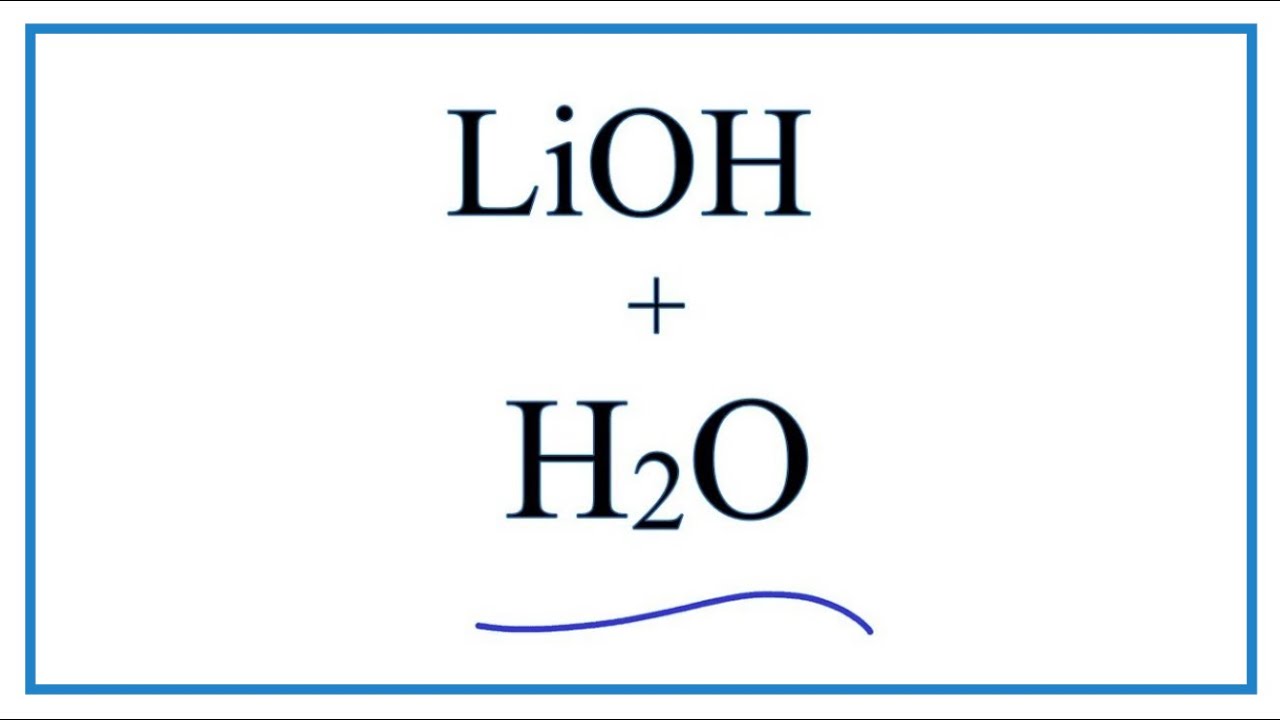
SOLVED: Predict the products of the following acidlbase reactions: LiOH (aq) HBrkoq) LiOH (aq) HBr(aq) noreaction LiOH (aq) HBrkaq) H,Od) + LiBr(aq) LiOH (aq) HBrkag) Li (aq) + OH" (aq) LiOH(aq) +
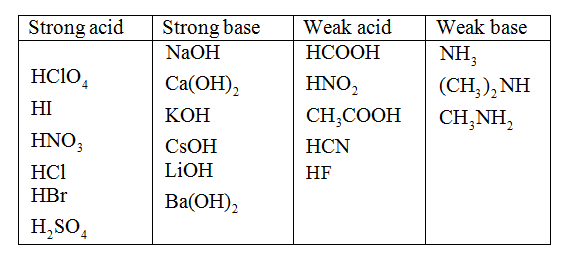
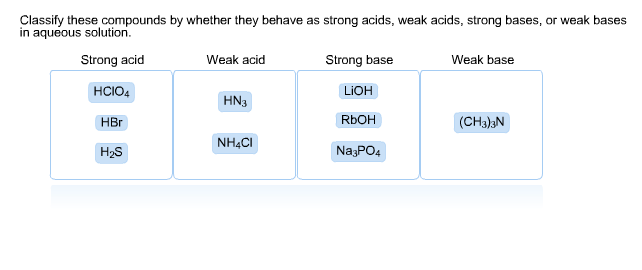
Classify each substance as a strong acid, strong base, weak acid, or weak base - Home Work Help - Learn CBSE Forum

Effect of initial acid and base concentration on generation of HCl and LiOH | Download Scientific Diagram

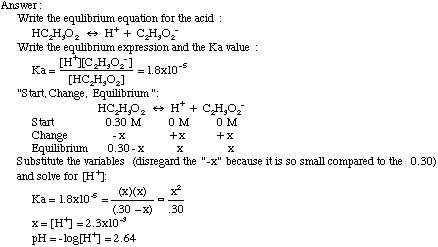
Equilibrium – Acids and Bases. Review of Acids and Bases Arrhenius Theory of Acids and Bases ▫An acid is a substance that dissociates in water to produce. - ppt download