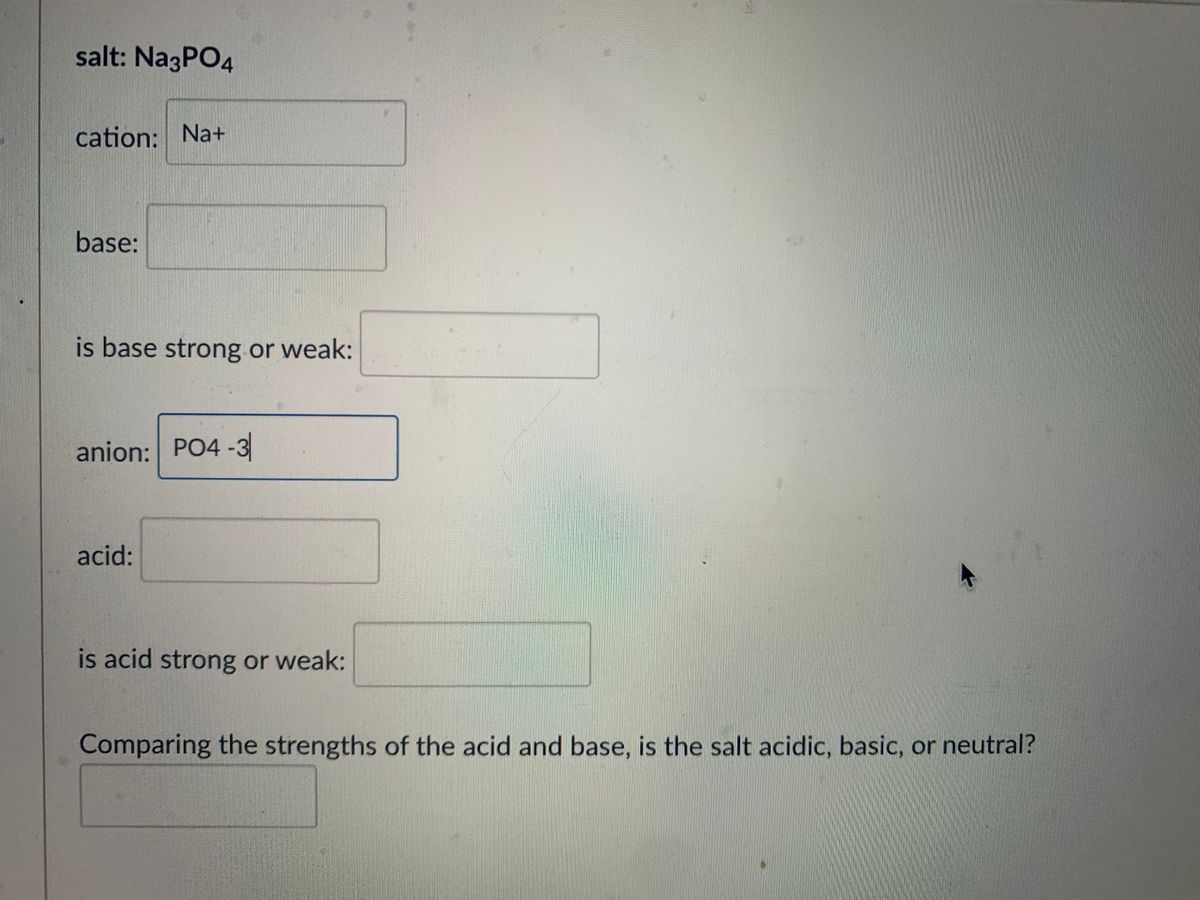
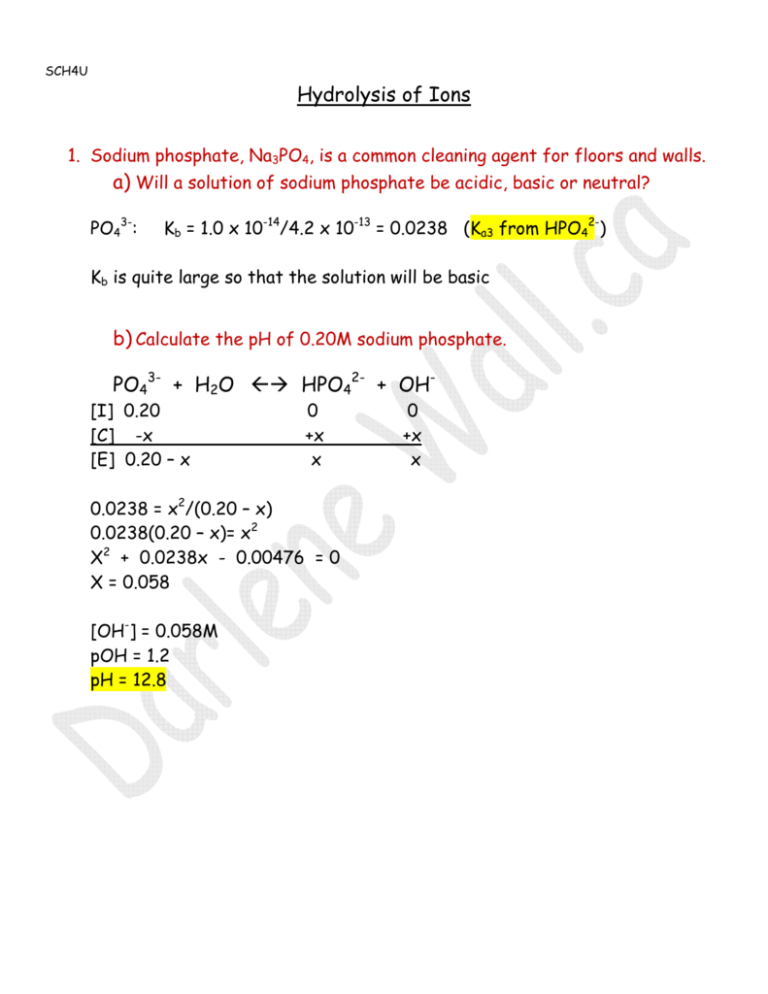
SOLVED: The household cleaner TSP contains sodium phosphate as the active ingredient: In solution the phosphate ion reacts with water as shown in the following equation. PO4(aq) H,O(aq) HPO4aq) + OH(aq) (a)
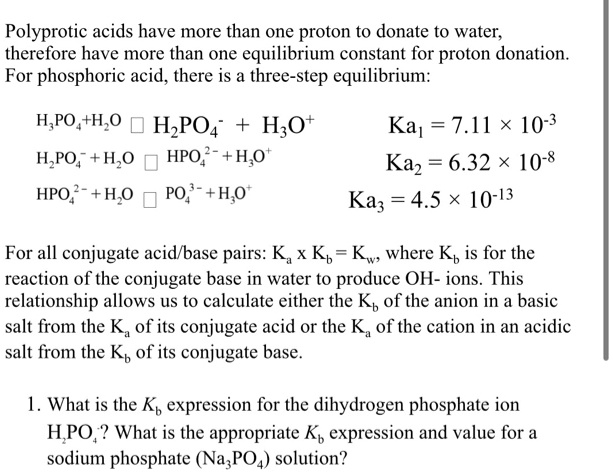
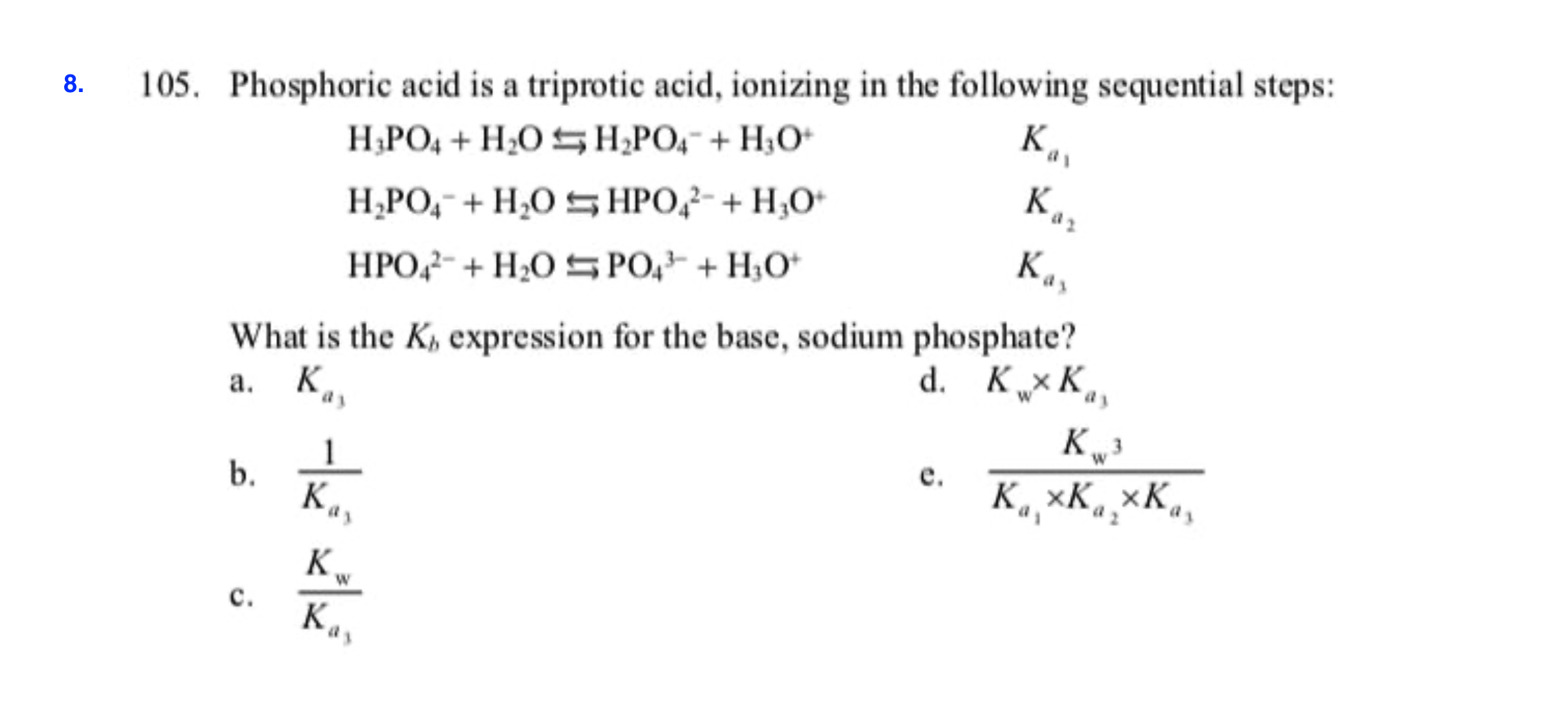
SOLVED: Polyprotic acids have more than one proton to donate to water; therefore have more than one equilibrium constant for proton donation For phosphoric acid, there is a three-step equilibrium: H,PO +H,o