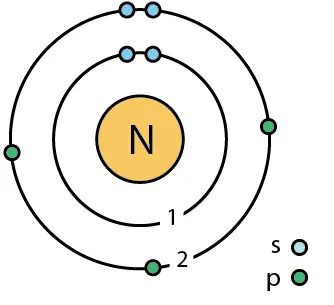
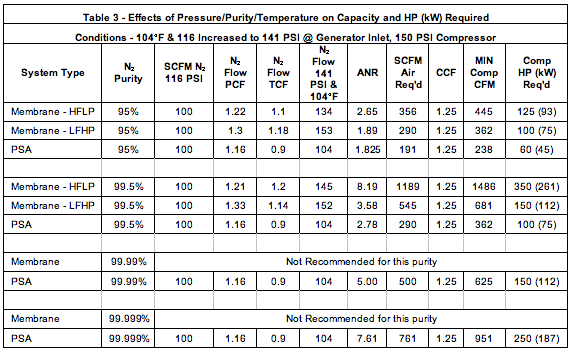
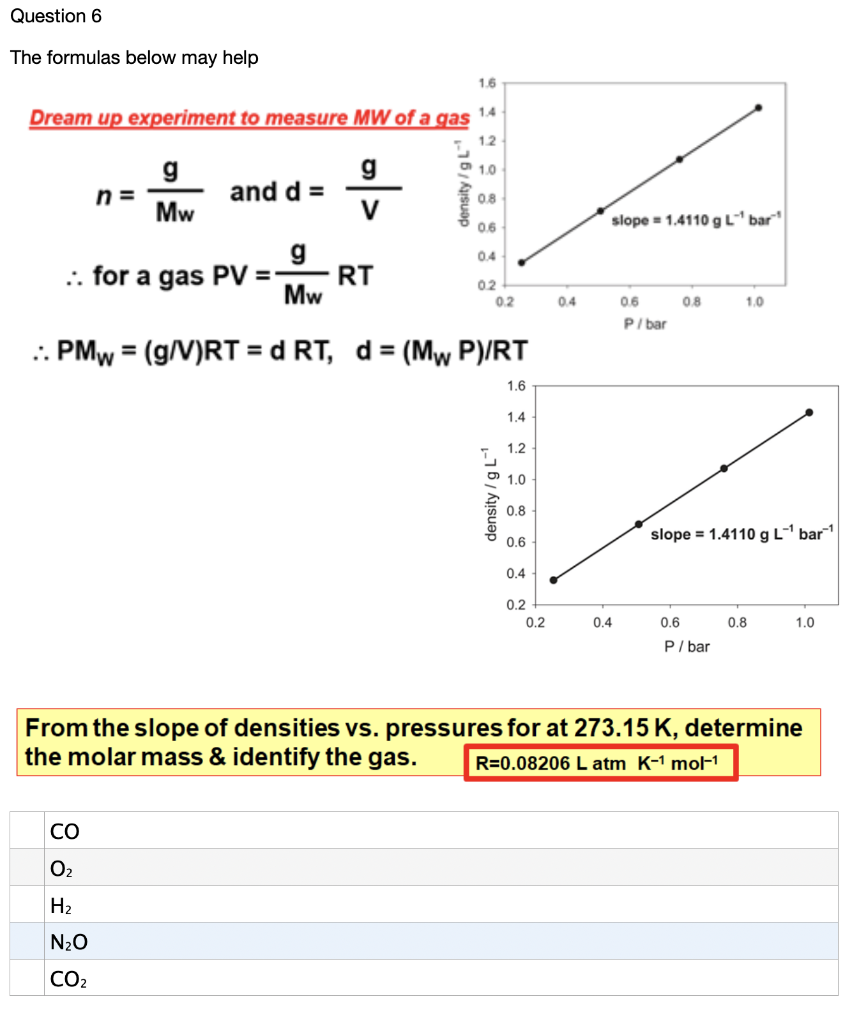
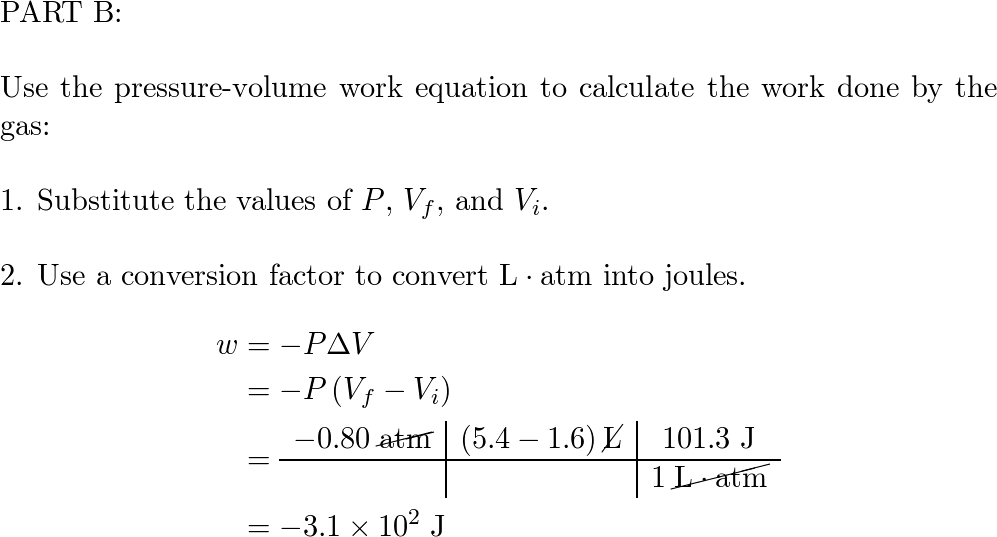
Q11 Calculate the Volume occupied by 5.25g of Nitrogen at 26 degree celcius and 74.2cm of pressure - YouTube
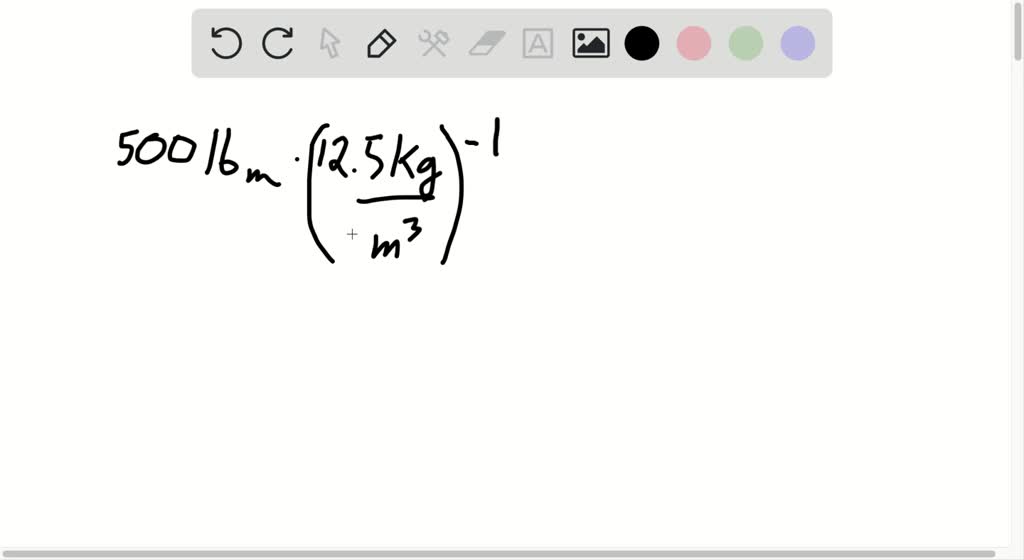
SOLVED:Five hundred 1 bm of nitrogen is to be charged into a small metal cylinder at 25^∘ C, at a pressure such that the gas density is 12.5 kg / m^3. Without